Version: 1.0
Date: Febraury 5, 2024
This publication is protected by copyright and can be used in accordance with the Creative Commons CC BY-NC-SA license. This license permits distributing, remixing, adapting, and building upon the material in any medium in any format for non-commercial purposes only as long as attribution is given to Queen’s University. If the material is remixed, adapted, or built upon, the resulting material must be licensed in accordance with the Creative Commons CC By-NC-SA license or under identical terms.
Inquiries and permission requests for commercial use may be directed to:
Queen’s University
Vice-Principal Research
Research, Compliance, Training, and Ethics
chair.greb@queensu.ca, hsreb@queensu.ca
Research Compliance, Training and Ethics
Purpose
The purpose of this guideline is to:
- Provide guidance on the requirements for submitting a medical case report study/series and chart reviews for research purposes.
Background
For HSREB, a case report study/series is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient/person.
For GREB, a case report is a detailed report of the situation, events, and intervention or support provided in a person's file.
Certain types of case report studies require REB approval.
Please note:
Queen’s REBs do not issue retroactive approval for applications (i.e., if a submission requires REB approval, the application is required to be submitted before the participant consents and any data are collected. If a submission requires REB exemption, it is preferred that the application is to be submitted before the participant consents but may be submitted after).
Types of case report studies that require REB approval
Research case report studies:
Research case report studies will be reviewed under the TCPS 2, Article 2.1.
A research case report satisfies the following:
- Includes a research objective and/or question (i.e., What, how, or why).
- Makes a conceptual and theoretical contribution to the discipline.
- This could include the development of a research instrument.
- It could have theoretical propositions.
- It will be submitted to an external publication or conference.
Chart review studies:
Chart review studies are a type of research in which pre-recorded, patient-centered data are used to answer one or more research questions. This type of study can be used to answer specific clinical questions in a less resource-intensive manner. A chart review has a clear objective and looks for trends or commonalities among participants’ charts.
A research case report and chart review studies are required to be submitted for approval from the REB before the case report/chart review begins (i.e. before consenting the participant).
Types of case report studies that do not require REB approval
Teaching case report studies:
Teaching cases are exempt from ethics review based on the TCPS2, Article 2.5. The ‘intent or purpose’ of a teaching case report study is for educational or learning purposes rather than research and, therefore, does not fall under the scope of the TCPS 2.
A teaching case report will satisfy the following:
- It would be written as a “story.”
- It would be written to support problem-based learning.
- It would require teaching notes.
- It could have theoretical propositions.
- It would value practical implications more than theoretical knowledge.
Decision Tree for determining what requires REB approval
Please see the decision tree below to determine if submission to the REB is required. Note: Queen’s
REBs will not issue a retroactive approval. If REB review is required, submission and approval by
the REB are required before the participant consents and data is collected. If REB exemption is
required, ideally this application is submitted prior to consenting and collecting data, but may be
submitted after for an exemption letter.
Any non-compliance will be referred to the research compliance and training team.
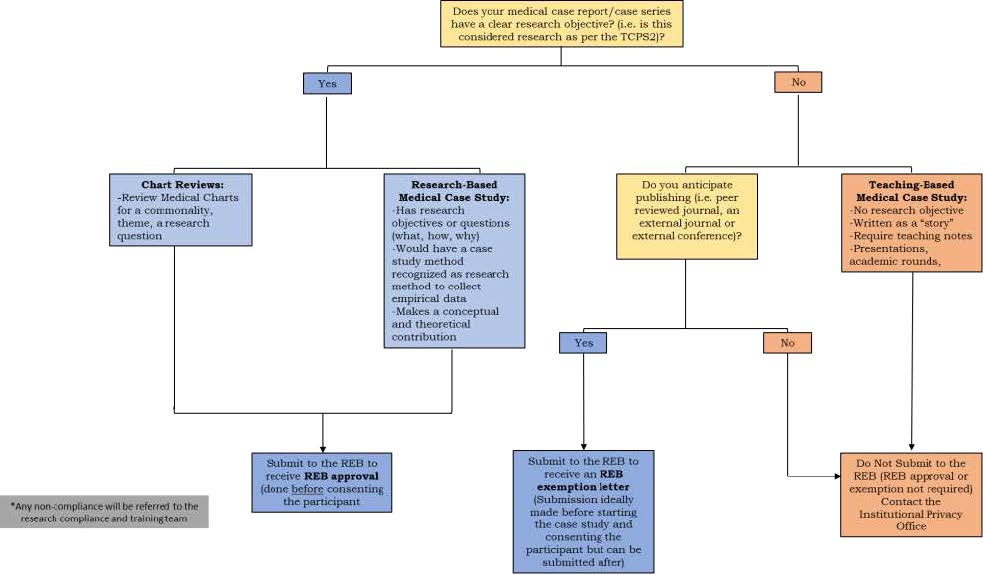
- Question: Does your medical case report/case series have a clear research objective? (i.e. is this considered research as per the TCPS2)?
- If YES
- Option 1 - Chart Reviews:
- Review Medical Chats for a commonality, theme, a research question
- Option 2 - Research-Based Medical Case Study:
- Has research objectives or questions (what, how, why)
- Would have a case study method recognized as research method to collect empirical data
- Makes a conceptual and theoretical contribution
- Action - Submit to the REB to receive REB approval (done before consenting the participant)
- Option 1 - Chart Reviews:
- If NO
- Option 1 - do you anticipate publishing (i.e. peer reviewed journal, an external journal or external conference)?
- If YES - Submit to the REB to receive an REB exemption letter (Submission ideally made before starting the case study and consenting the participant but can be submitted after)
- If NO - Do Not Submit to the REB (REB approval or exemption not required) Contact the Institutional Privacy Office.
- Option 2 - Teaching-Based Medical Case Study:
- No research objective
- Written as a "story"
- Require teaching notes
- Presentations, academic rounds
- Action - Do Not Submit to the REB (REB approval or exemption not required) Contact the Institutional Privacy Office.
- Option 1 - do you anticipate publishing (i.e. peer reviewed journal, an external journal or external conference)?
- If YES
*Any non-compliance will be referred to the research compliance and training team.
Critical considerations for research case report studies
- Consent will be obtained from the participant, parent/legal guardian/substitute decision maker. Use of the Queen’s REB consent form template for case studies is required. The case report consent form template will be used to obtain consent (a qualified physician must also sign the consent form if the report is being authored by students/residents/fellows).
- There is no intention to test various therapies/treatments/interventions prospectively or retrospectively.
- Assent will be obtained for those who can assent, where consent has already been obtained by the parent/legal guardian/substitute decision maker.
- The consent/assent process will be documented and kept on file for 5 years per Queen’s University guidelines.
- Justification of personal information/Personal Health Information (PHI) is required. It is best practice to limit the information collected (e.g., age, gender) (i.e., not using the full date of birth (DOB)/date of death (DOD) or any other information used in combination could lead to the identification of individuals). All measures will be taken to minimize the risk of re-identification through publication from the participant/friends/family members.
- All images/photographs will be de-identified (i.e., do not include name, medical record number, DOB) and do not include pictures with faces/facial features.
- The investigator, sub-investigators, or anyone connected to them through their interpersonal relationships (including their partners, family members, or former or current professional associates) will not receive any personal financial benefit from the report.
- Students/Residents/Fellows ensure that case reports are co-authored by a qualified physician who has appropriate credentials, is aware of, and shall make all reasonable efforts to comply with the applicable laws, guidelines, policies, and professional obligations.
- A TRAQ DSS form has been submitted to obtain hospital approval.
- Approval has been obtained from your departmental research committee.
- Refer to the CARE Guidelines and the CARE Checklist when writing your case report.
If you have any questions about your case report study/series, please get in touch with HSREB or GREB at HSREB@queensu.ca or chair.greb@queensu.ca
