FIG. 1.
Fine tuning of
protein concentration is not possible without X-chromosome dosage compensation.
Passage of
Y-chromosomes and X-chromosomes through the generations occurs either in male (M) or
female (F) cells. Contributions of chromosomes to the cytosolic protein concentration are
shown for autosomes, for X-chromosomes (grey), and for Y-chromosomes (vertical
stripes).
The contribution of the latter is actually much less than shown, so that halving the
contribution of the X-chromosome ("dosage compensation") in female generations
would keep the cytosolic protein concentration essentially independent of the sex of the host cell.
[This figure was not included in the original 1994 paper.] |
|
What is it about the intracellular environment that has to
be stabilized? Why is fine-tuning important? A possible answer was given previously when
addressing another paradox (Forsdyke, 1985, 1991,
1992). Cytotoxic T cells recognize
surface complexes of MHC class I proteins in association with peptides derived from
proteins synthesized within the same cell. Since mechanisms of self/not-self
discrimination appear to be extracellular, cells are held to load MHC proteins
intracellularly with peptides from both self
and not-self proteins for display at the cell surface.
Some MHC-self-peptide complexes are
indeed found (Rotzschke & Falk, 1991). However, the virtue of an obligatory display of
self peptides is not readily apparent; it requires the prior deletion or inactivation of
all T cells specific for MHC-self-peptide complexes, thus generating extensive "holes" in the T-cell repertoire
(Du Pasquier & Blomberg,
1982; Vidovik & Matzinger, 1988; Schild et al., 1990; Ohno, 1991). A mechanism
permitting some intracellular discrimination between self proteins and not-self proteins
would allow the preferential loading of MHC class I proteins with peptides derived from
not-self proteins, thus avoiding the logistic problem of competition with a myriad of
peptides derived from self proteins.
For a cell to distinguish intracellularly
between a self protein and a not-self protein (encoded by an intracellular pathogen),
would appear to be a formidable problem. Both classes of protein might, after all, be
synthesized on host ribosomes and might be released into similar cytosolic compartments.
However, there are two key differences which might be exploited by an appropriate
surveillance mechanism.
The first of these is that, relative to not-self protein encoding
genes, each self protein-encoding gene has had more evolutionary time to fine-tune its
product concentration to the intracellular environment created by the other self genes
with which it has been travelling through the generations.
The second is that, whereas self protein-encoding genes are
essentially at peace with the concentrations at which their products have arrived, the raison
d'etre of most foreign pathogens is, at some time within the lifespan of their host,
to increase in number. This may imply synthesizing specific proteins at rates resulting in
unacceptably high cytosolic concentrations.
If a pathogen with a short generation time and high mutation rate could gain a
foothold, it would readily accommodate to the first difference. However, the second
difference, combined with the heat-shock response (discussed elsewhere; Forsdyke, 1994;
1995), could be decisive.
5.
Differential Aggregation of Foreign Proteins
How would an "unacceptably
high" cytosolic concentration be detected? When macromolecules in solution
reach a critical concentration it becomes energetically more favourable for them to
aggregate, like-with-like, than to remain in simple solution. The aggregation involves a
liberation of bound water and an increase in entropy. Being primarily entropy driven, the
aggregation is promoted by an increase in temperature (Lauffer, 1975, 1989; Leikin &
Parsegian, 1992).
The crowded cytosol constitutes an environment which
readily drives proteins out of solution when they exceed individual concentration
thresholds, This is well recognized from the difficulties encountered when trying to
over-express proteins within foreign cytosols using expression vectors. In such systems
the formation of insoluble aggregates is greatly enhanced by increasing temperature over a
physiological range. This has been shown very clearly in a recent study of foreign protein
expression in mouse cells (Nguyen et al., 1989).
The proposal, then, is that not-self proteins more
readily "trip" the intracellular surveillance
system because their concentrations are not so fine-tuned to the phase-separating
proclivities of the crowded host cytosol as are the concentrations of self proteins (Fig.
2). In an organism without cytotoxic T cells, or their equivalent, this would trigger cell
death (Forsdyke, 1995). In an organism with T cells the aggregates would be directed to a
site for degradation to peptides. Specific peptides would then be displayed in association
with MHC class I proteins at the cell surface (Rotzschke & Falk,
1991). A cell would
thus become labelled for destruction by cytotoxic T cells (Forsdyke, 1995). An advantage
of this scheme is that it leaves open for an organism the option of declaring one of its
own self proteins as "foreign" should its synthesis
or turnover become disordered.
|

FIG. 2. Theoretical curves showing that most
gene products only limit phenotype at low concentrations, and that aggregation occurs at
concentrations exceeding that corresponding to two autosomal allelic genes or one
mammalian X-linked gene.
Although gene dosages are normally discrete, gene product
(protein) concentrations (circles) and phenotypes (triangles) are shown as
continuous functions. The situation prevailing in a normal cytosol is indicated by the
vertical arrow. In the absence of aggregation, the concentration of gene product is taken
to be a rectilinear function of the dosage (copy number) of a constitutive gene (filled
circles). Normally, a particular gene product only limits the phenotypic character to
which it contributes at low concentrations. As the normal cytosolic concentration is
approached other gene products become limiting (plateau in values of phenotypic character
assayed; filled triangles).
Thus, decreasing gene product concentration by half may not
change the phenotype. At above normal gene dosages, gene product aggregation occurs and
there is a fall in the quantity of gene product present in non-aggregated form (loss of
rectilinearity; open circles). These changes may have some effect on phenotype (fall in
phenotypic parameter assayed; open triangles), but the major effect will be the
"tripping" of the intracellular not-self surveillance system by aggregates. |
|
6. Role
of X Chromosome Inactivation
Without dosage compensation there would be a
fluctuation in the total intracellular protein concentration between male and female
generations. This would imply a fluctuation in the pressure to drive individual proteins
out of simple solution when their concentrations exceeded specific concentration
thresholds. An increase in the activity of the X chromosome in male generations
(Muller,
1948; Kuroda et al., 1991), or inactivation of one X chromosome in female
generations (Lyon, 1992), would stabilize the pressure and favour fine-tuning of
gene-product concentrations over evolutionary time.
A corollary of this is that, in
addition to being under evolutionary constraint to preserve specific function, genes encoding proteins are also under
evolutionary constraint both to maintain the collective pressure to drive individual
proteins from solution and to maintain individual protein solubilities in the face of that
collective pressure (Forsdyke, 1994b).
A disturbance of phenotype resulting from
hemizygosity enforced by deterioration of the Y chromosome could involve the specific functions of sex chromosome-encoded proteins
and/or their collective functions as
proteins per se. While the concentration of a protein in a heterozygote might
decrease to a level insufficient to affect specific function (i.e. the concentration of
the protein would still correspond to a point on the plateau of the dose-response curve;
Forsdyke, 1994), the decrease in concentration of the protein might itself be sufficient
to affect genetic fitness due to an "exceedingly minute",
but real, influence on some concentration-dependent collective protein function, such as
intracellular self/not-self discrimination. This would have driven the evolution of dosage
compensation and would appear to resolve Muller's paradox (Muller,
1948). Collective
functions less likely to be involved would include effects on the polymerization of
tubulin and actin, and the binding of ions (Donnan equilibrium).
7.
Two Active X Chromosomes in Oocytes and Embryos
No dosage compensation is evident in mammalian oocytes or in female
preblastula embryos (Lyon, 1992).
[But see Added
Note March 2009 at end of Aneuploidy paper.] The prolonged phase of X-chromosome decondensation in
meiotic oocytes probably relates to the need to reactivate the inactive X-chromosome
(resetting methylation patterns), and to correct DNA damage (Bernstein & Bernstein,
1991). This is obviously a very special case for which there should be mechanisms to
prevent inadvertent aggregation of X-encoded proteins.
The preblastula embryo is also a
special case. Even if some intracellular aggregation were to occur and peptide
presentation were possible in a preblastula embryo, at this stage of development the
differentiation of cytotoxic T cells would not have occurred. It is possible that
aggregation of an X-encoded regulatory protein in a female preblastula embryo would play a
critical role in switching off one of the X-chromosomes (Forsdyke,
1995).
8.
Compensation in Z/W Chromosome Systems
In some species (e.g. birds, butterflies, snakes),
the female is the heterogametic sex with Z and W chromosomes and the male is homogametic
(two Z chromosomes). Whereas in eutherian mammals the X-chromosome is about 5% of the
genome, the avian Z-chromosome is about 10%, which approaches the value for the fruit fly.
Thus, if compensation is good for mammals it would seem even more appropriate that
compensation exist in birds.
Dosage compensation in avian systems does not seem to follow
the mammalian model; there is no equivalent of the Barr body and both Z chromosomes
replicate together in males (Ohno, 1967). On the basis of very limited studies it has been
argued that species with Z/W chromosome systems are dosage tolerant (Johnson & Turner,
1979; Baverstock et al., 1982). However, these studies measured protein activities, not actual protein concentrations.
It has been
found that, even in the case of compensated genes, product activity levels may vary
between the sexes in different tissues, presumably in response to hormonal influences
(Steel & Midgeon, 1973). It is predicted that dosage compensation will be found in Z/W
chromosome-bearing species, possibly following the fruit fly model with increased
expression of the Z chromosome when in the heterogametic sex. The reason for this is that
intracellular self/not-self discrimination is a quite fundamental process conferring
resistance to infection and is likely to have evolved well before the evolution of sex
chromosomes (Forsdyke, 1985, 1991, 1992). Other predictions of the hypothesis are
presented elsewhere (Forsdyke, 1995).
|
The author thanks Dr Susamo Ohno for review of an early version
of the manuscript and the Medical Research Council of Canada and the Leukaemia Research
Fund of Toronto for support.
REFERENCES
BAVERSTOCK, P. R., ADAMS, M., POLKINGHORNE, R. W. & GELDER,
M. (1982). A sex-linked enzyme in birds: Z-chromosome conservation but no dosage
compensation. Nature, Lond 296, 763-766.
BERNSTEIN, C. & BERNSTEIN, H. (I 991). Aging, Sex and DNA Repair. New York: Academic Press.
BOOK, J. A. & SANTESSON, B. (1961). Nuclear sex in triploid
XXY human cells. Lancet 2, 318.
BULL, J. J. (1983). Evolution
of Sex-Determining Mechanisms. Menlo Park, CA: Benjamin-Cummings.
CHANDRA, H. S. (1985). Is human X-chromosome inactivation a
sex-determining device? Proc. natn. Acad Sci. U.S.A.
82, 6947-6949.
CHARLESWORTH, B. (1978). Model for evolution of Y-chromosomes
and dosage compensation. Proc. natn. Acad. Sci. U.S.A.
75, 5618-5622.
CHARLESWORTH, B. (1991). The evolution of sex chromosomes. Science 251, 1030-1033.
DU PASQUIER, L. & BLOMBERG, B. (1982). The expression of
antibody diversity in natural and laboratory-made polyploid individuals in the clawed toad
Xenopus. Immunogenetics 15, 251-261.
FORSDYKE, D. R. (1985). Heat shock proteins defend against
intracellular pathogens: a non-immunological basis for self/notself discrimination. J. theor. Biol. 115, 471473.
FORSDYKE, D. R. (1991). Early evolution of MHC polymorphism. J. theor. Biol. 150, 451-456.
FORSDYKE, D. R. (1992). Two signal model of self/not-self immune
discrimination: an update. J. theor. Biol.
154, 109-118.
FORSDYKE, D. R. (1994). The heat-shock response and the
molecular basis of genetic dominance. J. theor. Biol. 167,
1-5.
FORSDYKE, D. R. (1995). Entropy-driven protein self-aggregation
as the basis for self/not-self discrimination in the crowded cytosol: what the Greeks did
not know. J. Biol. Systems 3,
273-287.
HALDANE, J. B. S. (1933). The part played by recurrent mutation
in evolution. Am. Nat. 67, 5-19.
HARNDEN, D. G. (1961). Nuclear sex in triploid XXY human cells. Lancet 2, 488.
HODGKIN, J. (I 990). Sex determination compared in Drosophila
and Caenorhabditis. Nature, Lond. 344,
721 728.
JACOBS, P. A. & MIDGEON, B. R. (1989). Studies of
X-chromosome inactivation in trisomies. Cytogenet. Cell
Genet. 50, 75-77.
JOHNSON, M. S. & TURNER, J. R. G. (1979). Absence of dosage
compensation for a sex-linked enzyme in butterflies (Heliconius). Heredity 43, 71-77.
KURODA, M. I., KERNAN, M. J., KREBER, R.,
GANETZKY, B. & BAKER, B. S. (1991). The maleless protein associates with
the X-chromosome to regulate dosage-compensation in Drosophila. Cell 66, 935-947.
LAUFFER, M. A. (1975). Entropy-driven
Processes in Biology. New York: Springer-Verlag.
LAUFFER, M. A. (1989). Motion
in Biological Systems. New York: A. R. Liss.
LEIKIN, S. & PARSEGIAN, V. A. (1992). A
mechanism for temperature-favoured protein assembly. FASEB
J. 6, 418.
LINDSLEY, D. L., SANDLER, L., BAKER, B. S.,
CARPENTER, A.T., DENELL, R. E., HALL, J. C., JACOBS, P. A.,
MIKLOS, G. L., DAVIS, B. K., GETHMAN, R. C., HARDY, R.W., HESSLER,
A., MILLER, S.M., NOZAWA, H., PARRY, D. M. & GOULD-SOMERO, M.
(1972). Segmental aneuploidy and the genetic gross structure of the Drosophila genome.
Genetics 71, 157-184.
LYON, M. F. (1992). Some milestones in the history of
X-chromosome inactivation. A. Rev. Genet.
26, 17-28.
MULLER, H. J. (1948). Evidence on the precision of
genetic adaptation. Harvey Lect. 43,
165 229.
NGUYEN, V. T., MORANGE, M. & BENSAUDE, 0. (1989).
Protein denaturation during heat-shock and related stress. E. coli. beta-galactosidase
and Photinus pyralis luciferase inactivation in mouse cells. J. Biol. Chem. 264, 10487-10492.
OHNO, S. (1967). Sex
Chromosomes and Sex-Linked Genes. New York: Springer-Verlag.
OHNO, S. (1973). Conservation of ancient linkage groups in
evolution and some insights into the genetic regulatory mechanisms of X-inactivation. Cold Spring Harb. Symp. Quant. Biol. 38,
155-164.
OHNO, S. (1991). To be or not to be a responder in T-cell
responses: ubiquitous oligopeptides in all proteins. Immunogenetics
34, 215-221.
ORR, H. A. (1990). "Why polyploidy is rarer in
animals than plants" revisited. Am. Nat.
136, 759 770.
ROTZSCHKE, 0. & FALK, K. (1991). Naturally occurring
peptide antigens derived from the MHC class I-restricted processing pathway. Immun. Today, 12, 447 455.
SCHILD, H., ROTZSCHKE, O., KALBACHER, H. &
RAMMENSEE, H. G. (1990). Limit of T-cell tolerance to self proteins by peptide
presentation. Science 247, 1587
1589.
STEEL, M. W. & MIDGEON, B. R. (1973). Sex
differences in activity of glucose-6-phosphate dehydrogenase of cultured human foetal lung
cells despite X-inactivation. Biochem. Genet.
9, 163-170.
STEINEMANN, M. (1982). Multiple sex chromosomes in Drosophila
miranda: a system to study the degeneration of a chromosome. Chromosoma 86, 59-76.
VIDOVIK, D. & MATZINGER, P. (1988).
Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature, Land. 336, 222 -225.
 [ADDED NOTE:
2001. Strong evidence
for avian dosage compensation was reported by McQueen, H., McBride, D., Miele,
G., Bird, A. P., & Clinton, M. (2001) Dosage compensation in birds. Current
Biology 11, 253-257.
[ADDED NOTE:
2001. Strong evidence
for avian dosage compensation was reported by McQueen, H., McBride, D., Miele,
G., Bird, A. P., & Clinton, M. (2001) Dosage compensation in birds. Current
Biology 11, 253-257.
ADDED NOTE: 2005. See also
Cole, L. J. & Hollander, W. F. (1950) Hybrids of pigeon by ring dove. American
Naturalist 84,
275-307. In private correspondence with A. G. Cock (May
20th 1968) Hollander writes:
"For one locus in the pigeon and the dove (Streptopelia
risoria) we have a graded series of recessive mutant alleles which I
think meet your strict requirements.... The alleles d and
"blond" of pigeon and dove, respectively, are homologous as
shown by a hybrid test. "White" (= extreme dilution) in the
dove is a lower allele, and the heterozygous blond/white male is paler
than homozygous blond. Both in pigeon and dove the phenotypes of dilute
= blond females and homozygous males are indistinguishable. The example
is in my opinion a clear case of dosage compensation (sensu
strictu), but it has not been emphasized as such in the literature." |
[This is in the AGC
papers, deposited in 2008 in Queen's University Archives
Click Here
]
ADDED NOTE:
May 2008.
| Failure to inactivate an X chromosome
would disrupt the fine-tuning of intracellular protein concentrations
and could predispose females to autoimmune disease (see H. F. Pan et al.
2008 Medical Hypothesis 70,
1231-1232; D. L. Smith-Bouvier et al. 2008 J.
Exp. Med. 205, 1099-1108.). |
ADDED NOTE:
Sept 2008.
Following the full sequencing of two
avian genomes, further evidence for avian dosage compensation is
summarized by Arnold, Itoh and Melamed (2008 Annual
Review of Genomics and Human Genetics 9, 109-127).
Unlike most previous reviews, which dwell on mechanisms (the
"how" question), the authors discuss the selective pressures
that might have brought about the evolution of dosage compensation (the
"why" question). However, unaware of the hypothesis advanced
here, they provide an ad hoc "network" explanation in terms of
critical "hub genes" that regulate a multiplicity of other
genes. They rightly point to the fact that dosage compensation is not
normally brought about by variations in copy number of individual genes,
or even of small chromosomes, but
then continue:
"In contrast, monosomy or
trisomy for a whole large
chromosome usually presents a major problem; in humans, for
example, either is usually lethal. This is probably because
differences over a large genomic segment are more likely to
involve differences in dose of critical hub genes. The more hub
genes involved, the larger the problem. ... This view suggests
that the reduction in dose of a minority of X genes drove the
selection of complex molecular mechanisms to adjust X dosage in
one sex or the other." |
|
ADDED
NOTE: Nov 2008
| H-F. Pan and his
colleagues further marshal the evidence for failure to completely
inactivate one of the X chromosomes in SLE in a new article (Medical
Hypothesis 72, 99-109). The fact that failure to
inactivate an X chromosome in females would increase the aggregation
pressure is now acknowledged: "In these
females a higher than normal aggregation pressure would be expected." |
3. Aneuploid Lethality
Fine Tuning of
Intracellular Protein Concentrations, a Collective Protein Function Involved in Aneuploid Lethality, Sex-Determination and Speciation?
Journal of Theoretical Biology (1995) 172,
335-345.
(With copyright permission from Academic Press)
(Received on 26 January 1994, Accepted in revised form on 24 August 1994)
Abstract
1. Introduction
2. Aneuploid Lethality
2.1.
CUMULATIVE LOSS/GAIN OF MANY SEGMENTS REQUIRED FOR LETHALITY
2.2.
MANY CHROMOSOMAL ELEMENTS DETERMINE SEX AND DOSAGE COMPENSATION
2.3. X-CHROMOSOME DOSAGE
COMPENSATION
3. Aggregation Hypothesis
3.1.
SOME MUTATIONS AFFECT SPECIFIC PROTEIN CONCENTRATION, NOT FUNCTION
3.2.
FINE-TUNING OF INTRACELLULAR PROTEIN CONCENTRATIONS TO A MAXIMUM CONSISTENT WITH AVOIDING SELF-AGGREGATION
3.3. DOSAGE
COMPENSATION NECESSARY FOR FINE-TUNING
3.4. CELL
VOLUME AND FINE-TUNING IN DIFFERENT TISSUES
4. Implications of Hypothesis
4. 1.
TEMPERATURE SELECTIVELY DESTROYS MALE INTER-SEX CELLS
4.2.
DEPENDENCE ON X/A RATIO MEANS DEPENDENCE ON CONCENTRATION OF X-ENCODED PRODUCTS
4.3.
LESS AGGREGATION PRESSURE IN MALE ZYGOTES SWITCHES ON X HYPERTRANSCRIPTION
4.4.
SPECIATION AS A COLLECTIVE GENE FUNCTION. HALDANE'S RULE
4.5.
RECOMBINATION HELPS GENES PREDICT THEIR FUTURE ENVIRONMENT
4.6. FINE-TUNING IN ASEXUAL
SPECIES
End Note (Oct. 2008)
End Note (March 2009)
End_Note_(Oct_2010)
Abstract.
The assertion that sex-chromosome dosage compensation arose because aneuploidy for an
entire chromosome is lethal, begs the question of why aneuploidy is lethal. It has been
proposed that aneuploid lethality results from impairment of a collective protein function
(Forsdyke, 1994, J. theor. Biol. 167,
7-12).
Cytosolic
proteins, by virtue of their concentrations, exert a pressure tending to drive members of
individual protein species into self-aggregates. Other evolutionary time, each gene has
fine-tuned the concentration of its product to a maximum consistent with avoiding
self-aggregation in the crowded cytosol.
Because of this aggregation pressure and the
imprecision of their own fine-tuning, the proteins of members of other species, the
corresponding genes of which may have been transported to a cell as viruses (or gametes),
are specifically aggregated. The death of the cell and its enclosed virus results.
Aneuploidy impairs this process, with lethal consequences for the organism.
The hypothesis
leads to explanations for a variety of phenomena.
On the assumption that the concentration
of autosomal products determines cell volume, the observed dependence of sex-determination
on the ratio of X-chromosomes to autosomes is shown merely to be a dependence on the
concentration of the products of one X-chromosome.
The inviability of the heterogametic
sex among the offspring of an interspecies cross (Haldane's rule), follows from the
species-specific fine-tuning of the concentrations of X-chromosome encoded
products, relative to the concentration of autosomally encoded products.
|
|
1. Introduction
In species with sex chromosomes, one sex is usually
haploid with respect to one of the sex chromosomes. Thus, in humans and fruit flies, males
have only one copy of the X-chromosome (Jablonka & Lamb, 1990;
Charlesworth, 1991).
Since the concentration of a final gene product (usually a protein) is often directly
related to the number of gene copies, the concentration of X-chromosome gene products in
males should be half that in females. The process known as dosage compensation ensures
that this does not occur. In humans dosage compensation is achieved by inactivating one of
the X-chromosomes in females (Lyon, 1992). In the fruit fly Drosophila melanogaster
the transcriptional activity of the single male X-chromosome is increased (Muller, 1948;
Stern, 1960).
Muller's choice of the
value-laden
term "compensation" to describe the adjustment of
dosage implies that the process is advantageous. What adaptive advantage could dosage
compensation confer? The standard answer to this question begins by noting that, whichever
chromosome is affected, aneuploidy for more than 3% of a genome is usually
lethal. Since
the human X-chromosome is approximately 5% of the genome and the fruit fly X-chromosome is
approximately 20% of the genome, then without dosage compensation there might be an
intolerable degree of aneuploidy.
This answer was given by Muller & Kaplan
(1966), and
by Ohno (1967), and has been repeated in most subsequent reviews
(e.g. Baker & Belote,
1983; Jaffe & Laird, 1986). Certainly, mutations in genes affecting dosage
compensation are usually lethal (Lucchesi & Manning, 1987; Hodgkin,
1990). This
implies that aneuploidy for the X-chromosome would indeed be lethal. However, why aneuploid states are lethal is not explained.
If one accepts the deduction that X-chromosome
aneuploid lethality is just one example of the general problem of aneuploid lethality,
then two inductions can be made:
(i) Since X-chromosome aneuploidy in male fruit flies and humans can
be compensated by adjusting the concentrations of X-encoded gene products, then aneuploid
states, in general, may be lethal because of disturbances at the gene product level.
(ii) Since most of the X-chromosome is the subject of compensation,
then aneuploid states, in general, may be lethal because of a collective disturbance of
many
gene products. Thus, aneuploid lethality is
unlikely to occur because there is a small subset of genes which are aneuploid sensitive.
In this paper I first review evidence supporting these
inductions, derived from studies in three areas;
I then review a recently proposed mechanism by which gene products
could act collectively to produce lethal effects (Forsdyke,
1995). Finally, some
implications for the chromosomal determination of sex, and interspecies incompatibility at
the intracellular level, are discussed.
2.
Aneuploid Lethality
2.1. CUMULATIVE LOSS/GAIN OF MANY
SEGMENTS REQUIRED FOR LETHALITY
Lindsley and co-workers
(1972) studied segmental
aneuploidy in fruit flies. Small chromosome segments were either deleted to make a region
haploid, or duplicated to make the region triploid. Aneuploidy for only one locus was
found to be lethal. This was the triplolethal locus, which appears to encode an RNA
helicase (Dorer et al., 1990). In this case, neither the haploid state nor the
triploid state was tolerated. This would explain why the loss or gain of a DNA segment
containing triplolethal would not be accepted, but it would not explain aneuploidy
as a general phenomenon. In general, segments could be removed or duplicated with no
serious impairment of function. A collective
loss/gain of many segments was
necessary for lethality. Lindsley and co-workers concluded that:
"The deleterious effects of aneuploidy are, in the main,
caused by the additive
effects of genes that slightly reduce
viability, and not by the individual effects of a few aneuploid-lethal genes among a large
array of dosage-insensitive loci" (my
italics).
2.2. MANY CHROMOSOMAL ELEMENTS DETERMINE SEX AND DOSAGE
COMPENSATION
Another line of evidence in support of the above
inductions derives from studies of the determination of sex and dosage compensation in
certain polyploid variant organisms. These studies show that sex chromosome dosage
compensation depends, not on the absolute number of X-chromosomes, but on the X/A ratio.
In fruit flies, both sex and dosage compensation are determined by the X/A ratio. In
humans, only dosage compensation is determined by the X/A ratio (Book &
Santesson,
1961; Harnden, 1961; Mittwoch, 1973; Webb et al., 1992).
In 1983 Baker & Belote concluded that the X/A
ratio "remains the most enigmatic aspect of the hierarchy
controlling sex and dosage compensation". In spite of dramatic advances in our
understanding of the molecular basis of the implementation
of the information provided by the X/A ratio, a decade later the enigma remains. It
has been argued that a limited number of X-encoded proteins, (such as sis-a,
runt and the
helix-loop-helix protein sis-b), may be sufficient to act as numerators,
and that a limited number of autosomally encoded proteins (such as the helix-loop-helix
protein deadpan) may be sufficient to act as denominators
(reviewed by McKeown & Madigan,
1992). These arguments do not take into account the
historical evidence for a great multiplicity of numerator/denominator
elements.
Dependence of sex determination on the X/A ratio has
been described for the fruit fly (Bridges,
1925).
-
A ratio equal to or less than 0.5
generates a male (chromosome complement designated X,2A if the Y-chromosome is ignored).
-
A
ratio equal to or more than 1.0 generates a female (2X,2A).
-
A triploid individual (3X,3A)
has a ratio of 1.0 and is female.
-
A triploid individual with only one X-chromosome
(1X,3A)
has a ratio of 0.33 and is male.
-
A triploid individual with two X-chromosomes
(2X,3A),
however, has a ratio of 0.67. Such "intersexes"
have varying proportions of male and female cells, each cell type being found in course
patches indicating an early random commitment of individual cells to become clones of a
particular sex (Sanchez & Nöthiger,
1983).
2.2.1. Numerator elements
The addition of small segments of the X-chromosome to
2X,3A zygotes, prior to the time the male/female decision is made, should increase the
proportion of female cells. This was indeed found (Dobzhansky & Schultz, 1934; Baker
& Belote, 1983). Two remarkable features of these experiments were that:
(i) Most small segments of the X-chromosome were
equal
with respect to their abilities to increase the
proportion of female cells.
(ii) Increasing the length of the segment increased the proportion of female cells.
Thus the numerator element in the
X/A ratio appeared as a collective function
of X-chromosome segments and, by implication, of their genes and gene products.
2.2.2. Denominator elements
Baker & Belote
(1983) were able to conclude only that:
"The autosomal [denominator] component(s) whose dosage is
important for the primary determination of sex and/or dosage compensation has eluded
detection".
Little progress has been made in subsequent fruit fly studies
(Younger-Shepherd et
al., 1992). However, studies in human trisomies provide some evidence that the
denominator element in the X/A ratio determining dosage compensation might also be
a
collective function (Jacobs & Midgeon,
1989).
In normal human males (designated
X,2A if we ignore
the Y-chromosome), there is one active X-chromosome which [for
convenience] can be
regarded, by analogy with the fruit fly, to be permanently "hypertranscribed"
[relative to those of the female Xs if the latter were fruit fly
Xs], so that its products
"balance" those of the autosomes. Thus, the ratio
of X-encoded products to autosome-encoded products can be designated as 1.0.
In normal human females
(2X,2A) one X is inactive and replicates late in S-phase. The
other X is "hypertranscribed" as in the male
(giving a product ratio of 1.0). In tetraploid females (4X,4A) there are
two late-replicating X-chromosomes and two "hypertranscribed"
X-chromosomes whose products would thus balance those of the four sets of autosomes
(product ratio again 1.0).
In triploids
(3X,3A) there are two cell populations
reflecting two options, neither of them perfect. If there are two "hyperactive" X-chromosomes (and one inactive) then the level
of X-chromosome products is set to the level required to balance the products of four, not
three autosomes (predicted product ratio 1.33). If there is one "hyperactive" X-chromosome (and two inactive) then the level of
X-chromosome products is set to the level required to balance the products of two, not
three autosomes (predicted product ratio 0.67).
Jacobs and Midgeon asked whether there is one
powerful denominator autosome, or whether the denominator function is a collective
function of more than one autosome. They found, in individual human females, each trisomic
for a particular autosome, no cells in which the presence of the extra autosome would
allow both X-chromosomes to remain active. The study was unsatisfactory for various
reasons, particularly the fact that spontaneous trisomies for four of the 22 possible
autosomes were not found. Nevertheless, the data were consistent with the hypothesis that
the denominator function is collective. The authors wondered whether compensation
involves:
". . the action of a specific autosomal gene(s), or does it
depend on a more indirect attribute,
such as an increase in
nuclear volume ... ?" (my
italics).
In the same vein, Dobzhansky and Schulz wrote in 1934:
"One may, perhaps, account for the sex-determining function
of the autosomes even without assuming any autosomal sex genes. Sex may be determined by
the ratio between the number of X-chromosomes present in the cell and the size of the
cell. Cell size in most organisms (including Drosophila), is positively correlated
with the volume of chromosomal material contained in the nucleus."
It should be noted that hyperploidy is usually associated with an increase in cell volume
(Dobzhansky, 1929; Tal, 1980;
Lucchesi & Manning, 1987), and hypoploidy with a decrease
in cell volume (Bridges, 1939). This would appear to reflect the general dependence of
protein quantity on gene dosage. A change in volume would accommodate a change in the
quantity of an intracellular protein while maintaining its concentration constant
(Colclasure & Parker, 1991). This is an important point to which we will be returning.
2.3.
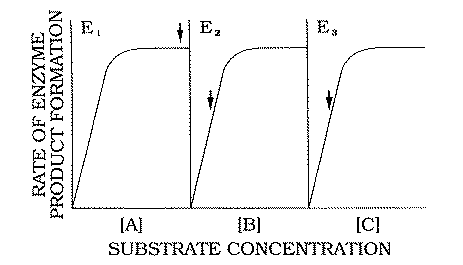
X-CHROMOSOME DOSAGE COMPENSATION
Many genes encode proteins with dosage sensitive
functions, such as enzymes. Typically, with increasing enzyme concentration there is a
progressive increase in the quantity of enzyme product formed. The quantity of this
product may be reflected in the phenotype. However, above a certain enzyme concentration
some other factor becomes rate-limiting (e.g. substrate availability); curves plotting the
rate of product formation as a function of enzyme concentration then tend to plateau.
2.3. 1. Dosage imbalance does not impair
specific protein functions
Muller (1948) was struck by the fact that
the
concentrations of most enzymes found within diploid cells correspond to a point well along
the plateau of the dose-response curve. In most cases, halving the concentration of the
enzyme would still generate a product level corresponding to the plateau of the
dose-response curve (Forsdyke,
1994a). Thus, there would be no phenotypic difference
between a female fruit fly cell containing two active X-chromosomes and a male fruit fly
cell containing one uncompensated
X-chromosome. Muller's paradox is that dosage compensation has evolved when there would
appear to be only, in his words, "exceedingly minute
differences" between compensated and uncompensated phenotypes.
2.3.2 Dosage imbalance impairs collective protein
function?
It has been proposed that the paradox would be
resolved if the pressure to evolve dosage compensation involved selection acting, not on
the unique individual phenotypes characteristic of each individual gene product, but on a
phenotype generated collectively by all X-encoded gene products (Forsdyke,
1994b).
The
Donnan equilibrium is an example of a collective protein function. Intracellular proteins
at physiological pH are usually charged molecules which can influence the equilibrium
distribution of ions across protein-impermeable membranes (Hitchcock,
1924). Over the
range of protein concentrations found in cells, the strength of the Donnan effect is
directly proportional to the quantity of intracellular protein. There is not an
intracellular protein concentration beyond which the effect no longer increases. If the
X-chromosome (20% of the genome) were not hyperactivated in the male fruit fly, then male
cytoplasm would have approximately 90% of the protein concentration of the female
cytoplasm. A difference of this magnitude is not "exceedingly
minute". It might adversely affect the Donnan equilibrium and hence
negatively influence phenotype.
However, the Donnan effect would appear to be
volume-sensitive. Adjusting cell volume should correct a concentration difference between
proteins in male and female cells. That this may not be feasible becomes apparent on
considering a novel collective function of intracellular proteins -- this is discussed
below.
3.
Aggregation Hypothesis
3.1. SOME MUTATIONS AFFECT SPECIFIC PROTEIN CONCENTRATION, NOT
FUNCTION
Some gene mutations affect the functions of unique
gene products. Other mutations affect the concentrations of those products, often without
influencing function per protein molecule. For example, the concentration of a protein
might be changed if the concentration of its mRNA were changed, either by a promoter
mutation affecting the transcription rate, or by a mutation in the 3' non-coding region
affecting mRNA stability. Similarly, a mutation in a protein encoding region might affect
protein stability, but have no effect on function per protein molecule.
As discussed above, within cells the intracellular
concentration of most proteins corresponds to a point well along the plateau of the plot
of activity (phenotype) against protein concentration. When mutations occur resulting in a
specific decrease in concentration of
a protein, the protein concentration will move to the left, corresponding to points on the
plateau of the dose-response curve.
Eventually a point will be reached where specific
function will begin to be impaired. There will then be an effect on phenotype and a
decrease in fitness. A detailed discussion of this, in the context of the evolution of
genetic dominance, is presented elsewhere (Forsdyke,
1994a). The basic point being made
here is that there is a lower limit below which a mutation-driven decrease in
concentration of a gene product cannot occur without affecting specific function.
By the same token, when mutations which specifically
increase the concentration of a
particular protein occur, the protein concentration will move to the right corresponding
to points on the plateau of the dose-response curve. In this case, there is no obvious
change in specific phenotype. For a given intracellular protein, does the actual
concentration found among members of a biological species fluctuate between the lower
limit, defined above, and an upper limit set simply by the solution limit? Alternatively,
are there further selective forces which determine a precise concentration setting for
each protein?
3.2. FINE-TUNING OF INTRACELLULAR PROTEIN CONCENTRATIONS TO A
MAXIMUM CONSISTENT WITH AVOIDING SELF-AGGREGATION
Generally, it is easier for a protein to remain
soluble in a salt solution at physiological pH than to remain soluble in the "crowded cytosol"
(Fulton, 1982). The solubility of a
particular protein species is determined by the concentrations of the other proteins that
accompany it (Lauffer, 1975). It has been proposed that, over evolutionary time, each
individual gene fine-tunes the concentration of its product to the concentrations of the
products of the other genes with which it has been travelling through the generations
(Forsdyke, 1995).
Irrespective of its specific function, the "aim"
of each gene is to maximize the concentration of its own products. Collectively, the
products of all genes exert a pressure tending to drive individual protein species into
aggregates if they exceed their solubility limit. Each individual protein species both
contributes to the pressure and is acted upon by the pressure (Fig. 1).
|

| FIG. 1. What point on the dose-response curve
corresponds to the normal concentration of a gene product within a cell?
This hypothetical
in vivo dose-response curve shows some quantitative measure of phenotype (e.g. rate
of formation of enzyme product) as a function of the concentration of a gene product which
contributes to that phenotype (such as the concentration of an enzyme). The phenotypic
parameter increases with gene dosage until point A when some other factor (e.g.
substrate availability) becomes rate-limiting. The curve then plateaus. B
corresponds to the minimum concentration of gene product required for a "margin
of safety", so that heterozygote function is not impaired (Forsdyke, 1994a).
E corresponds to the concentration at which the gene product would still be soluble
if no other proteins were present. Above this concentration, the protein would
self-aggregate and the phenotypic parameter would decrease. D corresponds to the
concentration at which aggregation would occur in the presence of cytosolic proteins
(Forsdyke, 1995). The horizontal arrows symbolize the aggregation pressure exerted
collectively by cytosolic proteins, which tends to push the descending limb of the
dose-response curve to the left.
Thus, it would seem that the concentration of a protein
in cells of different members of a species could fluctuate between points B and D
with no effect on phenotype. It is proposed, however, that over evolutionary time genes
have "fine-tuned" the concentrations of their products to a
maximum consistent with avoiding self-aggregation. This point might correspond to C
(marked by a vertical arrow), which is slightly to the left of D, thus providing a
small "margin of safety" against inadvertent self-aggregation. |
|
The selective force leading to the evolution of this
pressure involves the invasion of the intracellular space by a member of another species
of organism (e.g. a virus), which has not had the opportunity to fine-tune so precisely
the concentrations of its gene products. As discussed elsewhere (Forsdyke, 1985, 1991,
1995), by mechanisms involving heat-shock proteins, proteins encoded by virus genes would
tend to be specifically aggregated. Irrespective of any effect on specific function, this
aggregation would trip an output system which, depending on the organism, would involve
cell death inflicted either directly, by an intracellular mechanism (e.g. apoptosis), or
indirectly, by cytotoxic T cells. A key feature of the aggregation is that it is primarily
entropy-driven and thus is favoured by a small increase in temperature (Lauffer,
1975).
The death of the cell and the pathogen it contains would usually be beneficial to the
organism. In some cases aggregation alone might suffice to limit the spread of the
pathogen.
3.3. DOSAGE COMPENSATION NECESSARY FOR FINE-TUNING
The need for fine-tuning over evolutionary time
provides a clear rationale for the evolution of dosage compensation (Forsdyke,
1994b).
Without dosage compensation, the cytosolic protein concentration would fluctuate between male and female generations.
Fine-tuning would be possible only for genes encoded by Y-chromosomes, which are eternally
locked in male generations. Failure to fine-tune would impair intracellular self/not-self
discrimination and thus increase the vulnerability of an organism to intracellular
pathogens.
|
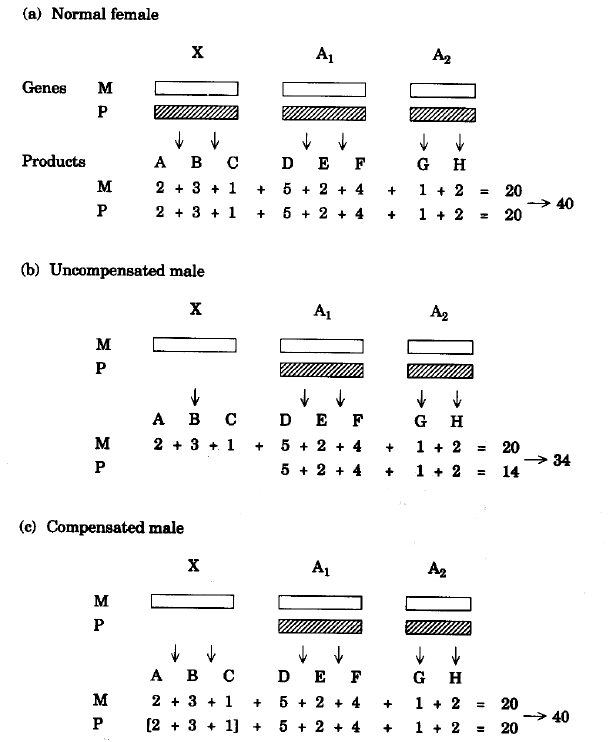
| FIG. 2.
Evolutionary fine-tuning of intracellular protein concentrations as an explanation for
X-chromosome dosage compensation.
Rectangles refer to maternal (M) and paternal (P)
haploid chromosome complements which combine to form either (a) a female zygote, (b) an
uncompensated male zygote, or (c) a compensated male zygote. Gene products (proteins) are A,
B and C (for the X-chromosome), D, E and F (for
autosome A1), and G and H (for autosome A2). The specific function of these proteins is not relevant to the
primary establishment of the X/A signal governing the initiation of X hypertranscription
in male fruit fly (dosage compensation).
The numbers below each protein indicate its
quantity, which has been fine-tuned over evolutionary time to a maximum consistent with
remaining in solution in the crowded cytosol. Numbers in brackets are the quantities of
products contributed by the maternal X-chromosome, which balance the products of the
paternal autosomes.
The proteins, by virtue of their concentrations, exert a collective
pressure (which can be expressed as the sum of individual protein concentrations), tending
to drive individual protein species into self-aggregates. For female zygotes and
compensated male zygotes the net aggregation pressure might be 40 units
per cell. For an uncompensated male zygote the net aggregation pressure would be only 34
units/cell.
Cell volume cannot be decreased by a factor of 34/40 in male
generations to maintain the aggregation pressure because the quantities of autosomal
products (D-H) are already fine-tuned to a maximum consistent with remaining
soluble in female generations. Since a high aggregation pressure is needed for the
detection of proteins encoded by intracellular pathogens, the viability of uncompensated
phenotypes will be decreased. |
|
Figure 2 shows a model of how this might work. It is
postulated that, without dosage compensation, the discrepancy in cytosolic protein
concentrations between male and female generations would not readily be corrected by an
intergenerational adjustment in cell volume. Reducing cell volume in male generations
[by
a factor of 34/40 in Fig. 2(b)], would slightly concentrate the products
of autosomal genes. Since these had already been fine-tuned in female generations to
maximize the solubility of each individual protein species, a further concentration might
trigger their aggregation. Intergenerational volume adjustment would work only by
compromising the degree of fine-tuning in female generations.
It can be argued that the lower concentration of
X-encoded products would slightly decrease the net pressure tending to drive the
autosomally encoded proteins into aggregates, and this might make it possible to
accommodate autosomal proteins in a smaller volume without triggering their aggregation.
However, it is postulated here that this would not be a feasible alternative. Cell volume
is autosomally determined. Experimental evidence to the contrary would falsify this aspect
of the hypothesis.
3.4. CELL VOLUME AND FINE-TUNING IN DIFFERENT TISSUES
It is apparent that the general setting of cell
volume as a function of the number of chromosomes (see Section
2.2.2) is subject to
tissue-specific modification. The major cell of vertebrate lymphoid tissues, the small
lymphocyte, is an extreme example. The ratio of cytoplasm to nucleus is very low. This can
be regarded as an adaptation for circulating in the lymphatic and vascular systems from
which lymphocytes migrate to regions of inflammation. It can be assumed that the concentration of cytoplasmic proteins is no less than
in a cell with more cytoplasm.
It should also be noted that the differential gene
expression associated with cell specialization requires fine-tuning to a particular subset
of genes in each tissue, as well as to the more generally expressed "house-keeping" genes. This might require different final
concentrations of the same gene product in different tissues. In some cases this could
have been achieved by gene duplication so that the same protein could be expressed under
the influence of different promoters in different tissues.
As discussed elsewhere
(Forsdyke, 1995), it is
envisaged that, in the case of proteins whose concentrations fluctuate as part of
physiological processes, concentration fine-tuning would have been with respect to the
maximum concentration attained. Similarly, in the case of proteins that form
stoichiometric complexes with other proteins, the whole complex would have been treated as
a unit during evolutionary fine-tuning. In the case of reversibly modified proteins
(phosphorylation, glycosylation), concentration fine-tuning would be with respect to the
least soluble form of the protein.
4.
Implications of Hypothesis
4. 1. TEMPERATURE SELECTIVELY DESTROYS MALE INTER-SEX CELLS
The aggregation hypothesis makes numerous explicit
experimental predictions and reinterpretations of previous data (Forsdyke,
1995). One of
these is that male cells in fruit fly intersexes should be more vulnerable to high
temperatures than female cells in fruit fly intersexes. Cells of a triploid intersex fruit
fly (2X,3A) can have their X-chromosome transcribed either in the male mode (giving a
protein level which would balance that corresponding to four autosomes), or in the female
mode (giving a protein level which would balance that corresponding to two autosomes). The
relative X/A product ratios in each actual case would be 1.33 (male cell)
and 0.67 (female cell).
The excess of X-encoded products in male cells
would tend to aggregate, leading to the selective death of male cells. Since aggregation
is promoted by a small increase in temperature (Lauffer, 1975; Forsdyke,
1994a),
incubating triploid intersexes at high temperatures would enhance the death of male cells,
while having little effect on female cells in which the pressure to aggregate would be
below normal (normal corresponding to a relative product ratio of 1.0).
Evidence in support of this has been presented by Dobzhansky (1930) and Lauge
(1980).
4.2. DEPENDENCE ON X/A RATIO MEANS DEPENDENCE ON CONCENTRATION OF
X-ENCODED PRODUCTS
It was proposed above (Section 3.3) that the volume of a cell is directly related to the
quantity of autosomal products it
must contain. Assuming, for simplicity, a rectilinear relationship, cell volume
(V) is
equal to kAp, where
Ap is the quantity of autosomal products and
k is a constant. It
follows that the concentration of
X-encoded products (Xp /V where
Xp is the quantity of X-chromosome-encoded products), is proportional
to the ratio of the quantity of X-chromosome encoded products to the quantity of
autosomally encoded products. Thus:
Xp/V = Xp/kAp = (1/k)Xp/Ap.
In that the quantities of the products are proportional to the relative
gene dosages, it follows that the concentration of X-encoded products is proportional to
the X/A ratio (defined as the ratio of the number of X-chromosomes to the number of
autosomes). From this it can be concluded that the statement that the determination of sex
and/or dosage compensation is dependent on the X/A ratio (Bridges,
1925), may merely be
acknowledging a dependence on the concentration
of X-encoded products (Xp/V).
4.3. LESS AGGREGATION PRESSURE IN MALE ZYGOTES SWITCHES ON X
HYPERTRANSCRIPTION
Prior to the onset of dosage compensation, male
fruit-fly zygotes would experience a decline in aggregation pressure as X-encoded maternal
gene products decreased to be replaced by a zygote's own single X-encoded products
(Mahowold & Kambysellis,
1980). In female fruit fly zygotes the aggregation pressure
would be sustained because decreasing maternal X-encoded products would be replaced by the
products of both X-chromosomes of the zygote. The aggregation pressure in female cells
(Pf) can be
regarded as equal to k'[2Xc+Ac], where
Ac is the concentration of autosomal products,
Xc is the
contribution to the concentration of X-chromosome encoded products of one X-chromosome and
k' is a constant.
Similarly, the aggregation pressure in uncompensated
male cells (Pm) would be equal to
k'[Xc + Ac]. Then the difference in aggregation
pressure between males and females (Pf
- Pm) would be equal to k'Xc.
The latter includes the concentration of the extra set of X-chromosome encoded products
which, as argued in Section 3.3, would depend on the X/A ratio (since cell volume is held
to be autosomally determined). Thus, the switch to X hypertranscription would depend primarily on the collective
functioning of all X-chromosome encoded genes.
A secondary
effect of the greater pressure in female cells could be that the homo- or hetero-dimerization of helix-loop-helix sex-determining proteins, such as
daughterless
(maternally derived) and sis-b (zygotically-derived), would be promoted. The regulatory
activity of helix-loop-helix proteins is usually greatly affected by such aggregation
(Thayer & Weintraub, 1993). The helix-loop-helix protein aggregates might activate the
zygotic sex-lethal gene early promoter (carried by both X-chromosomes) thus
switching to the female pattern of development (Keyes et al.,
1992). In male
zygotes, the aggregation pressure would be less and helix-loop-helix protein aggregates
would tend to dissociate so that the single sex-lethal gene would be inactive. This
would remove the negative influence of the sex-lethal product on the autosomally
encoded genes which initiate hypertranscription of the male X-chromosome (Gorman et
al., 1993).
4.4. SPECIATION AS A COLLECTIVE GENE FUNCTION. HALDANE'S RULE
A biological species can be viewed as having set up
intracellular barriers against members of other species, be they intracellular pathogens,
or gametes of a species related enough to show no prezygotic isolation (Templeton, 1989;
Coyne, 1992). The fine-tuning of cytosolic protein concentrations, continuing through the
generations and communicated to all interbreeding members of the species, would have
created an intracellular environment hostile to the gene products both of foreign
pathogens, and of the gametes of other species (Forsdyke,
1995).
A failure of members of a species to interbreed,
owing to some form of allopatric or sympatric isolation, would provide an opportunity for
mutational differences to arise between alleles that might affect the abilities of their
products to contribute to the cytosolic aggregation pressure. This might occur before any
allele-specific differences in phenotype could arise. Fusion of gametes from such
reproductively isolated lines would expose a cytoplasmic incompatibility owing to
differences in the fine-tuning of cytosolic protein concentrations. Thus, the list of
potential cytoplasmic incompatibilities between organisms should include "warfare", not only between organelles, as is widely recognized
(Hurst, 1993), but also between cytosolic proteins
(Forsdyke, 1995). The latter might have
played a role in the origin of species.
The particular vulnerability of members of the
heterogametic sex among the offspring of interspecies hybridizations (Haldane's rule), may
serve to illustrate the latter point. The cytosolic concentrations of proteins encoded by
one X-chromosome are uniquely fine-tuned to the concentrations of proteins encoded by its
haploid autosomal complement. In female fruit fly, each X is accompanied by at least one
compatible autosome complement. In male fruit fly, the solitary X is hypertranscribed and
only one X is accompanied by a compatible autosomal complement.
This could result in
changes in net aggregation pressure with adverse consequences for the organism. Figure 3
shows a simple model of how this might work. Any
X-linked gene which mutated so that the concentration of its product changed would suffice
to begin the isolation process. There would be no distinct "speciation
genes".
|

FIG. 3. Evolutionary fine-tuning of gene product
concentrations as an explanation for decreased viability of the heterogametic sex among
the offspring of interspecies crosses (Haldane's rule).
Following the convention of Fig.
2, at the top are the genomes of two wild-type members of a species. During
gametogenesis,
one produces a concentration deficiency mutant (-1 unit) for an X-chromosome
encoded gene
(mutant 1). The potential contribution to the net aggregation pressure thus falls from 20
to 19 units. This decrease in aggregation pressure might be corrected in female zygotes in
one step if a second gamete happened to have a perfectly matching concentration-excess
mutant (+1 unit). The figure shows correction in two steps.
The partially compensated
decrease in aggregation pressure (39.5 units) in the organism formed by mutants
1 and 2,
is tolerated for a sufficient number of generations for a second concentration excess
mutant to arise (mutant 3) to finally rebalance the genome. However, the relative balance
between X-chromosome encoded products and autosomally encoded products would have been
changed. A back-cross with the original wild type would generate females with normal
aggregation pressures, but, owing to hypertranscription of the solitary X-chromosome in
the heterogametic sex, there would be an imbalance in males. |
|
The idea that Haldane's rule reflects an
X-chromosome/autosome incompatibility is an old one (Dobzhansky,
1937). However, studies
of hybrids between different fruit fly species have been interpreted by Coyne (1985) as
supporting an alternative X-chromosome/ Y-chromosome incompatibility theory. Wu (1992)
noted that Coyne only considered hybrid sterility.
Recently, Orr (1993) provided evidence that hybrid non-viability
reflects an X-chromosome/autosome incompatibility, in keeping with the arguments
presented here.
The founders of the "modern
synthesis" proposed that speciation required the divergence of many "ordinary" genes. Thus, speciation is a collective gene
function (Dobzhansky, 1937; Muller,
1942). Although there are some special cases, in
general, subsequent work has supported the hypothesis (Coyne,
1992). For example, at least
150 genes are implicated in the inviability of hybrids between various grasshopper races
which are believed to have arisen quite recently (Barton & Hewitt,
1981). The role of
a possible "speciation gene" involved in hybrid
male sterility in fruit fly (Orr, 1992), remains to be determined.
I present evidence elsewhere that fine-tuning
occurring at the DNA level ("GC/AT pressure"), may
also contribute to speciation (Forsdyke,
1994c).
4.5. RECOMBINATION HELPS GENES PREDICT THEIR FUTURE ENVIRONMENT
A major theme of this paper is that alleles compete
to get into the next generation, not only on the basis of differences in the specific
functions that they encode, but on the basis of their abilities to be "good mixers" and fit in with other genes in contributing to
collective functions, particularly the function concerned with generating a cytosolic
aggregation pressure. Recombination cross-over between genes serves this purpose in that
it separates emerging gene coalitions, thus effectively homogenizing the genome and
ensuring that each allele "knows" with some
certainty what aggregation pressure its products can expect to encounter in the next
generation (Dawkins, 1990).
4.6.
FINE-TUNING IN ASEXUAL SPECIES
In both sexual and asexual species an ability to
aggregate specifically proteins encoded by an intracellular pathogen would appear
advantageous. The effectiveness of this would depend on the effectiveness of the
fine-tuning of intracellular protein concentrations. Since the pressure to aggregate would
be greatly affected by ambient temperature (Lauffer,
1975), homeothermic species would be
able to fine-tune more precisely than poikilothermic species. Homeothermy is encountered
in sexual species, but many sexual species are also poikilothermic.
In sexual species, because of the "horizontal" mingling of genomes, mutations affecting the
concentration of a protein can be compensated by other mutations and can spread (Fig. 3).
In asexual species, a genomic response to an adverse mutation requires a transmission
"vertically" through the generations until
compensating mutations can occur (Muller,
1932). Thus, fine-tuning in asexual organisms
might depend more on the direct elimination of concentration mutants, rather than on the
emergence of compensating mutants.
|
This work was supported by the Leukaemia Research Fund
(Toronto), the Canadian Medical Research Council and the School of Graduate Studies of
Queen's University. I thank Mr. H. Metz for assistance with the figures.
REFERENCES
BAKER, B. S. & BELOTE, J. M. (1983). Sex determination and
dosage compensation in Drosophila melanogaster. A. Rev.
Genet. 17, 345-393.
BARTON, N. H. & HEWITT, G. M. (1981). The genetic basis of
hybrid inviability in the grasshopper Podisma pedestris. Heredity
47, 367-385.
BOOK, J. A. & SANTESSON, B. (1961). Nuclear sex in triploid
XXY human cells. Lancet, 2,
318.
BRIDGES, C. B. (1925). Sex in relation to chromosomes and genes.
Am. Nat. 59, 127-137.
BRIDGES, C. B. (1939). Cytological and genetic basis of sex. In:
Sex and Internal Secretions, 2nd
edn (Allen, E., ed.) pp. 15-63. Baltimore: Williams and Wilkins.
CHARLESWORTH, B. (1991). The evolution of sex chromosomes. Science 251, 1030-1033.
COLCLASURE, G. C. & PARKER, J. C. (1991). Cytosolic protein
concentration is the primary volume signal in dog red cells. J.
gen. Physiol. 98, 881-892.
COYNE, J. A. (1985). The genetic basis of Haldane's rule. Nature, Lond. 314, 736-738.
COYNE, J. A. (1992). Genetics and speciation. Nature, Lond. 355, 511-515.
DAWKINS, R. (1990). Parasites, desiderata lists and the paradox
of the organism. Parasitol. 100,
S63-S73.
DOBZHANSKY, T. (1929). The influence of the quantity and quality
of chromosomal material on the size of cells in Drosophila melanogaster. Roux Arch. Entwickim. 115, 363-379.
DOBZHANSKY, T. (1930). Genetical and environmental factors
influencing the type of intersexes in Drosophila melanogaster. Am.
Nat. 64, 261-271.
DOBZHANSKY, T. (1937). Genetic nature of species differences. Am. Nat. 71, 404-420.
DOBZHANSKY, T. & SCHULTZ, J. (1934). The distribution of sex
factors in the X-chromosome of Drosophila melanogaster. J.
Genet. 28, 349-386.
DORER, D. R., CHRISTENSEN, A. C. & JOHNSON, D. H. (1990). A
novel RNA helicase gene tightly linked to the Triplolethal locus of Drosophila. Nucleic Acids Res. 18, 5489-5494.
FORSDYKE, D. R. (1985). Heat shock proteins defend against
intracellular pathogens: a non-immunological basis for self/not-self discrimination. J. theor. Biol. 115, 471-473.
FORSDYKE, D. R. (1991). Early evolution of MHC polymorphism. J. theor. Biol. 150, 451-456.
FORSDYKE, D. R. (1994a). The heat shock response and the
molecular basis of genetic dominance. J. theor. Biol.
167, 1-5.
FORSDYKE, D. R. (1994b). Relationship of X-chromosome dosage
compensation to intracellular self/not-self discrimination: a resolution of Muller's
paradox? J. theor. Biol. 167, 7-12.
FORSDYKE, D. R. (1994c). A stem-loop "kissing" model
for the initiation of recombination and the origin of introns. FASEB.
J. 81 A1395.
FORSDYKE, D. R. (1994d). Hierarchy of frequencies of
complementary trinucleotide pairs depends on percentage G + C: implications for
speciation. Proc. Can. Fed. Biol. Socs. 37,
152.
FORSDYKE, D. R. (1995). Entropy-driven protein self-aggregation
as the basis for inter-species discrimination in the crowded cytosol. J. biol. Systems 39, 273-287.
FULTON, A. B. (1982). How crowded is the cytoplasm? Cell 30, 345-347.
GORMAN, M., KURODA, M. I. & BAKER, B. S. (1993). Regulation
of the sex-specific binding of the maleless dosage compensation protein to the male
X-chromosome in Drosophila. Cell 72,
39-49.
HARNDEN, D. G. (1961). Nuclear sex in triploid XXY human cells. Lancet 2, 488.
HITCHCOCK, D. I. (1924). Proteins and the Donnan equilibrium. Physiol. Rev. 4, 505-531.
HODGKIN, J. (1990). Sex determination compared in Drosophila
and Caenorhabditis. Nature, Lond. 344,
721-728.
HURST, L. D. (1993). The incidences, mechanisms and evolution of
cytoplasmic sex-ratio distorters in animals. Biol. Rev.
68, 121-193.
JABLONKA, E. & LAMB, M. J. (1990). The evolution of
heteromorphic sex chromosomes. Biol. Rev.
65, 249-276.
JACOBS, P. A. & MIDGEON, B. R. (1989). Studies of
X-chromosome inactivation in trisomies. Cytogenet. Cell
Genet. 50, 75-77.
JAFFE, E. & LAIRD, C. (1986). Dosage compensation in Drosophila.
Trends Genet. 2, 316-321.
KEYES, L. N., CLINE, T. W. & SCHEDL, P. (1992). The primary
sex determination signal of Drosophila acts at the level of transcription. Cell 68, 933-943.
LAUFFER, M. A. (1975). Entropy-Driven
Processes in Biology. New York: Springer-Verlag.
LAUGE, G. (1980). Sex determination. In: The Genetics and Biology of Drosophila, Vol.
2d. (Ashburner, M. & Wright, T. R. F., eds) pp. 33-106. New York: Academic Press.
LINDSLEY, D. L., SANDLER, L., BAKER, B. S., CARPENTER A. T.,
DENELL, R. E., HALL, J. C., JACOBS, P. A., MIKLOS, G. L., DAVIS, B. K., GETHMAN R. C.,
HARDY, R. W., HESSLER, A., MILLER, S. M., NOZAWA, H., PARRY, D. M. & GOULD-SOMERO, M.
(1972). Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics 71, 157-184.
LUCCESI, J. C. & MANNING, J. E. (1987). Gene dosage compensation in Drosophila
melanogaster. Adv. Genet. 24, 371-429.
LYON, M. F. (1992). Some milestones in the history of X-chromosome inactivation.
Annu. Rev. Genet. 26, 17-28.
MAHOWOLD, A. P. & KAMBYSELLIS, M. P. (1980). Oogenesis. In: The Genetics and Biology of Drosophila, Vol.
2d. (Ashburner, M. & Wright, T. R. E., eds) pp. 141 224. New York: Academic Press.
MCKEOWN, M. & MADIGAN, S. J. (1992). Sex determination and differentiation
in invertebrates: Drosophila and Caenorhabditis elegans. Curr.
Opin. Cell Biol. 4, 948-954.
MITTWOCH, U. (1973). The Genetics of Sex
Differentiation. New York: Academic Press.
MULLER, H. J. (1932). Some genetic aspects of sex. Am.
Nat. 64, 118-138.
MULLER, H. J. (1942). Isolating mechanisms, evolution and temperature. Biol. Symp. 6, 71-125.
MULLER, H. J. (1948). Evidence on the precision of genetic adaptation. Harvey Lect. 43, 165-229.
MULLER, H. J. & KAPLAN, W. D. (1966). The dosage compensation of
Drosophila and mammals as showing the accuracy of the normal type. Genet. Res. 8. 41-59.
OHNO, S. (1967). Sex chromosomes and
Sex-Linked Genes. New York: Springer-Verlag.
ORR, H. A. (1992). Mapping and characterization of a "speciation
gene"in Drosophila. Genet. Res. 59,
73-80.
ORR, H. A. (1993). Haldane's rule has multiple genetic causes. Nature, Lond. 361, 532-533.
SANCHEZ, L. & NOTHIGER, R. (1983). Sex determination and dosage compensation
in Drosophila melanogaster: production of male clones in XX females. Eur. molec. Biol. J. 2, 485-491.
STERN, C. (1960). Dosage compensation: development of a new concept and new
facts. Can. J. Genet. Cytol. 2,
105-118.
TAL, M. (1980). Physiology of polyploidy. In: Polyploidy:
Biological Relevance (Lewis, W. H., ed). pp. 61-133. New York: Plenum
Press.
TEMPLETON, A. R. (1989). The meaning of species and speciation. A genetical
perspective. In: Speciation and its Consequences
(Otte, D. & Endler, J. A., eds) pp. 3-27. Sunderland, Ma: Sinauer Associates.
THAYER, M. J. & WEINTRAUB, H. (1993). A cellular factor stimulates the
DNA-binding activity of MyoD and E47. Proc. natn. Acad.
Sci. U.S.A. 90, 6483-6487.
WEBB, S., DE VRIES, T. J. & KAUFMAN, M. H. (1992). The differential staining
pattern of the X-chromosome in the embryonic and extra-embryonic tissues of
postimplantation homozygous tetraploid mouse embryos. Genet.
Res. 59, 205-214.
Wu, C. I. (1992). A note on Haldane's rule: hybrid inviability versus hybrid
sterility. Evolution 46, 1584-1587.
YOUNGER-SHEPHERD, S., VAESSIN, H., BIER, E., JAN, L. Y. & JAN, Y. N. (1992).
Deadpan, an essential pan-neural gene encoding an HLH protein, acts as a
denominator in Drosophila sex determination. Cell 70,
911-922.
End Note (Oct. 2008)
"Exaggeration"
Calvin Bridges noted (1922;
American
Naturalist
56, 51-63) that one copy aneuploidy was lethal for fruit fly chromosomes 2 and 3 (both large), but not for
chromosome 4 (small) or the large X chromosome (as in normal males).
Nevertheless, in "haplo-IV" individuals many characters were
changed. In "haplo-X" individuals (i.e. normal males) there
were changed characters related to sexual dimorphism and some sex-linked
characters. In the case of recessive genes on the IVth chromosome in
"haplo-IV" individuals, there was a different "grade
of development" compared with the corresponding "diplo-IVs"
with two copies of the recessive gene. This phenomenon he named "exaggeration." He pointed out
that, paralleling this, "there is a class of sex-linked characters
[e.g. eosin eye and bobbed] that are exaggerated in the absence of one X
chromosome." He concluded:
"There is one striking
difference between haploidy for the X and haploidy for an
autosome - namely, that the changes connected with haploidy for
autosomes are relatively more numerous and extreme. Haploidy for
the second or third autosomes probably produces changes so great
as to be lethal, while haploidy for the very small
fourth-chromosome produces changes comparable in extent to those
of the male aside from the reproductive organs. The proportion
of sex-limited mutant characters in only about a tenth of the
total, while X contains about a quarter of the genes [of the
organism]. Since the changes in character produced by absence of
an X are relatively small, the internal
balance of the X must be relatively high." |
Doris Bachtrog (2008;
Genetics
180, 1123-29) considered that this represents the first discovery of
the phenomenon that Muller came to call "dosage
compensation."
|
End
Note (March 2009) Two Levels of Compensation
In 2006 microarray expression of
transcripts indicated that compensation was at two potentially
independent levels. Balanced expression was maintained between:
(1)
X chromosome and
autosomes (independently of sex).
(2)
The sexes (classical dosage compensation).
Thus, when haploid, basal expression of both the X chromosome and an
autosome are the same (say
one unit). When diploid, the
autosome remains
unchanged (basal) and the total
expression is two units (since there are now two
of each autosome). But the solitary X in males and the active
X in females (the one that is not turned off under classical dosage
compensation) are both hypertranscribed for a total expression of
two
units, thus balancing the autosome. (Among themselves the
autosomes are held to be balanced - the total expression of each
autosome pair being two units.) This new finding tends to support the
arguments raised in these pages, although we do not know to what extent
transcript levels and protein product levels are related.
(Nguyen & Disteche (2006) Nature
Genetics 38, 47-53; Gupta et al. (2006) J.
Biology 5, 3). |
End Note (Oct 2010) How to Tolerate Aneuploidy
If there is an extra copy of a chromosome (aneuploidy) then protein
concentration will tend to increase, with potentially toxic effects.
The idea that the negative effects of aneuploidy are due to changes
in intracellular protein concentrations (failure to dosage compensate),
predicts that mutations in mechanisms that regulate protein concentrations
may help an organism (or a cancer cell) to tolerate aneuploid states.
Ubiquitination marks proteins for proteosomal degradation. Torres et al.
(2010) report that proteosomal degradation is accelerated when a a
deubiquinating enzyme is mutated. This facilitates tolerance of aneuploid
states. Furthermore:
| "Twenty strains of budding yeast, each
bearing an extra copy of one or more ... yeast chromosomes
(henceforth disomic yeast strains), display decreased fitness ...
and a gene expression signature known as the environmental
stress response (Gasch et al., 2000). These ... traits
are due to the additional gene products produced from the
additional chromosomes. Primary aneuploid mouse cells exhibit
similar phenotypes (Williams et al., 2008). On the basis of these
findings, we propose that aneuploidy leads to an 'aneuploidy
stress response.' In this response, cells engage protein
degradation and folding pathways in an attempt to correct protein
stoichiometric imbalances caused by aneuploidy." |
Torres, E. M. et al. (2010) Identification of aneuploidy-tolerating
mutations. Cell 143, 71-83. |
Go to: X Chromosome Dosage Compensation
and Autoimmune Disease (Click Here)
Return to: X Chromosome
Dosage Compensation Index (Click Here)
Return to: Evolution Index
(Click Here)
Return to: Home-Page (Click Here)
This page was established circa 1998 and last edited
14 Oct 2010
by Donald R. Forsdyke