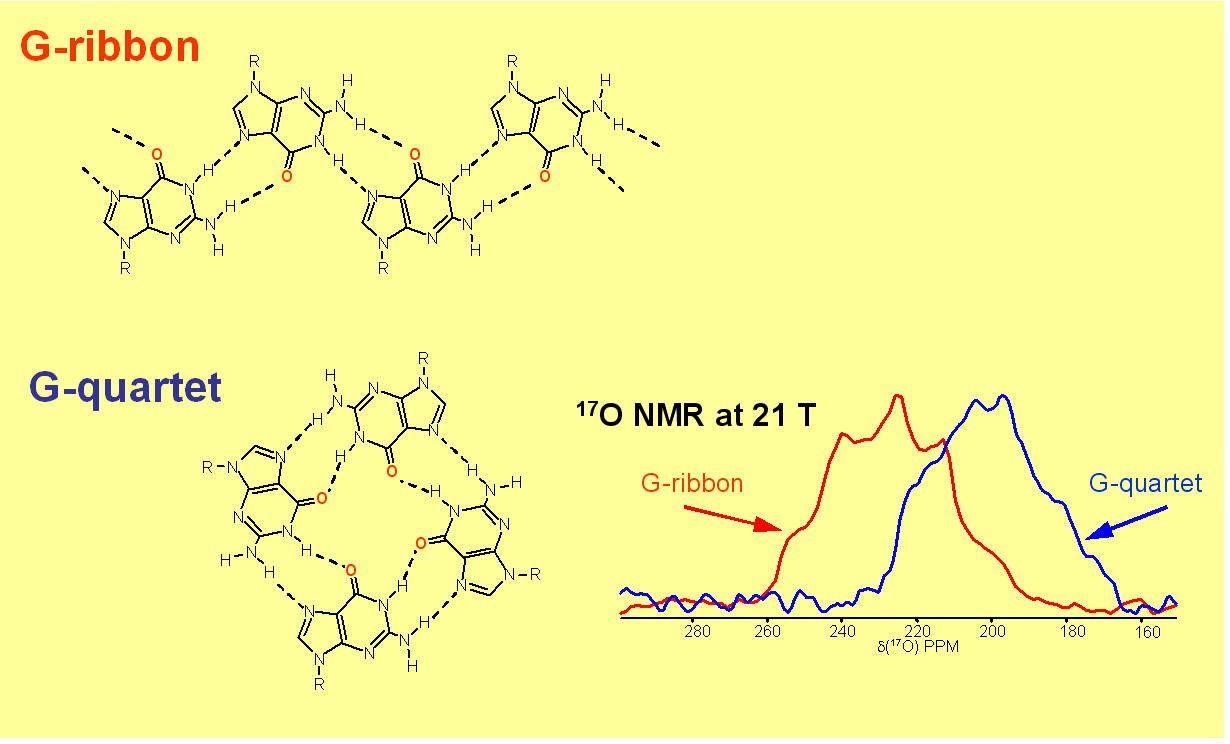
DNA oligomers containing repeats of guanine (G) can fold into four-stranded structures called G-quadruplexes. In the human genome, there are several G-rich regions where a G-quadruplex structure may potentially form. Among them, the telomere found at the termini of eukaryotic chromosomes is of particular importance. During normal cell division, telomeric DNA sequences shorten incrementally and such a cumulative loss eventually becomes lethal to somatic cells. An enzyme known as telomerase is capable of rebuilding the ends of telomeres. More importantly, this enzyme is found active in 85-90% of proliferating tumour cells but inactive in somatic cells. In the past decade, a tremendous amount of efforts have been devoted by scientists to the understanding of the relationship between cell immortalization in tumour cells and the maintenance of telomere length.2 Because the G-quadruplex structure may be a key feature of telomeres in vivo, it is of fundamental importance to understand all aspects of G-quadruplex formation and its binding to proteins.
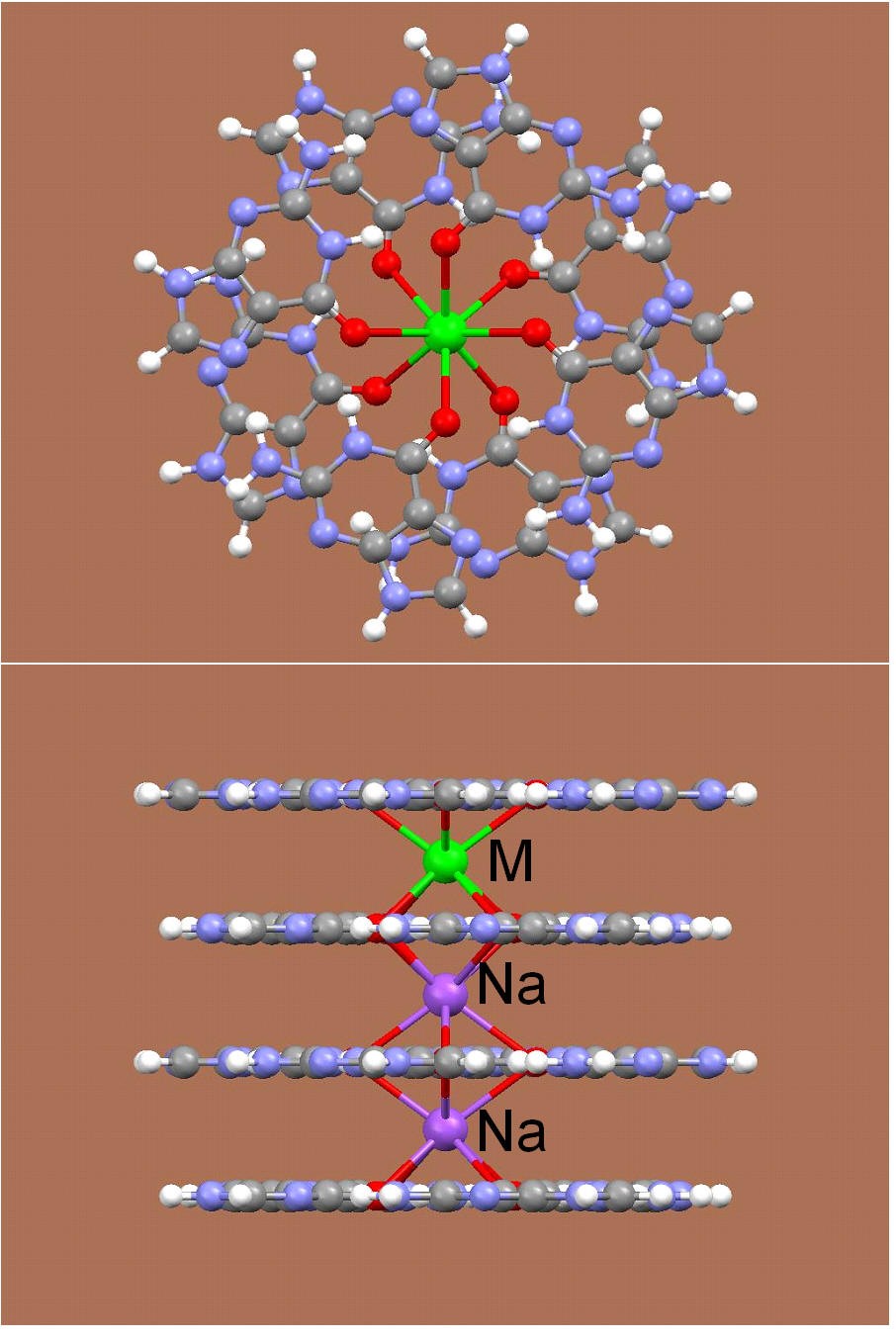
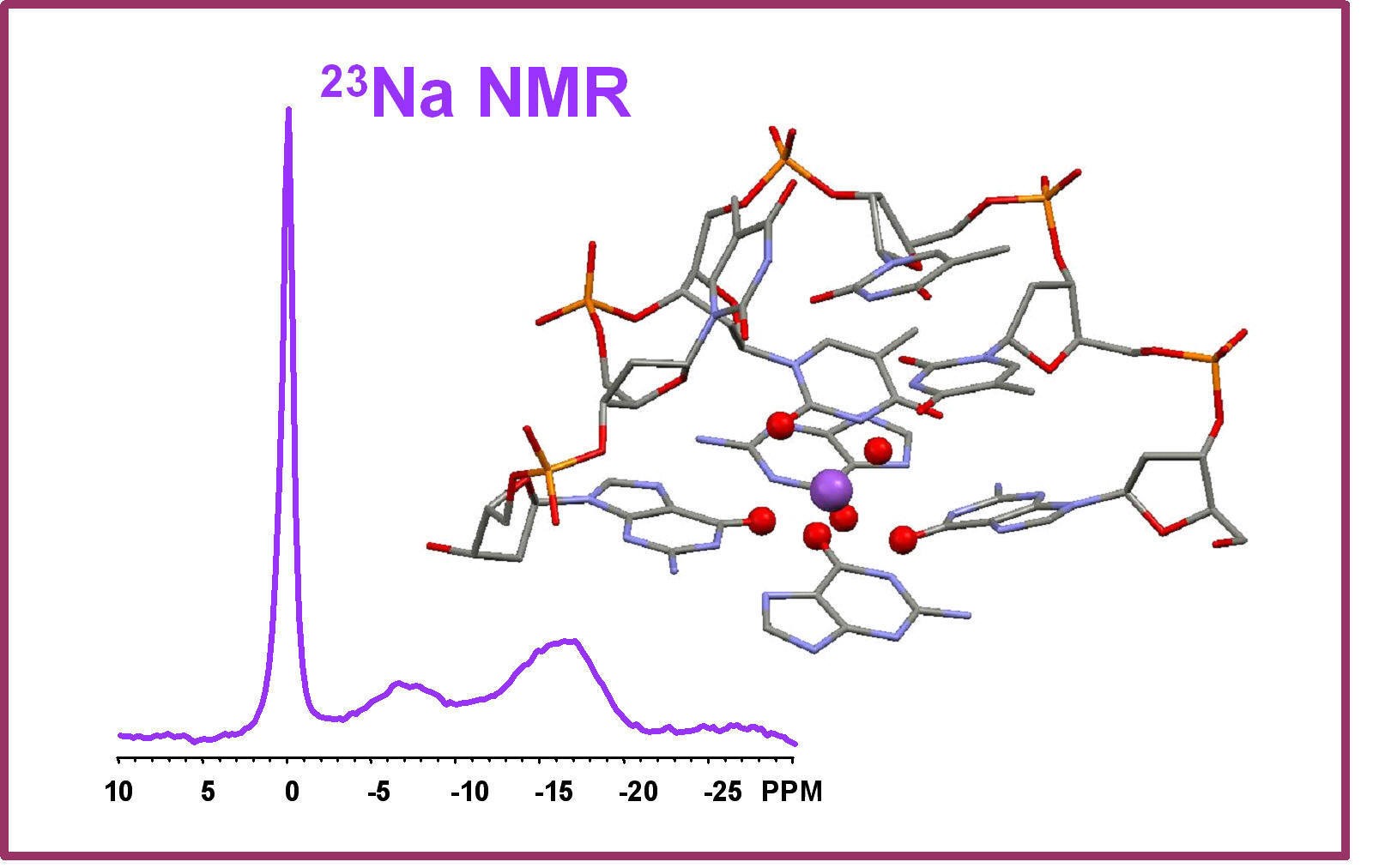
It is known that the presence of alkali metal ions such as K+ and Na+ is critical in the formation, stability and function of G-quadruplex DNA structures. Structural details regarding the mode of ion binding in G-quadruplex DNA have come primarily from X-ray crystallographic studies. Recently, we have made a breakthrough in this area. In particular, we have successfully identified the 23Na, 39K, 87Rb and 43Ca NMR spectral signatures for Na+, K+, Rb+ and Ca2+ ions bound to the G-quadruplex structure. We have applied this new solid-state NMR method to determine the number and coordination of Na+ ions in a telomeric DNA sequence from Oxytricha nova, d(G4T4G4). This work represents the first time that a technique other than crystallography has yielded site-specific information about Na+ ion coordination in G-quadruplex DNA. Currently, we are extending our novel NMR methodologies to study telomeric DNAs and their protein complexes.
Our publications in this area
- R. Ida and G. Wu, Journal of the American Chemical Society 130, 3590-3602 (2008).
- I. C. M. Kwan, A. Wong, Y.-M. She, M. E. Smith, and G. Wu, Chemical Communications 682-684 (2008). (Featured on the front cover)
- R. Ida, I. C. M. Kwan, and G. Wu, Chemical Communications 795-797 (2007).
- A. Wong, R. Ida and G. Wu, Biochemical and Biophysical Research Communications 337, 363-366 (2005).
- R. Ida and G. Wu, Chemical Communications 4294-4296 (2005).
- A. Wong, R. Ida, L. Spindler, and G. Wu, Journal of the American Chemical Society 127, 6990-6998 (2005).
- G. Wu and A. Wong, Biochemical and Biophysical Research Communications 323, 1139-1144 (2004).
- A. Wong and G. Wu, Journal of the American Chemical Society 125, 13895-13905 (2003).
- G. Wu, A. Wong, Z. Gan, and J. T. Davis, Journal of the American Chemical Society 125, 7182-7183 (2003).
- A. Wong, J. Fettinger, S. L. Forman, J. T. Davis, and G. Wu, Journal of the American Chemical Society 124, 742-743 (2002).
- G. Wu and A. Wong, Chemical Communications 2658-2659 (2001).
Oxygen is one of the most important elements in organic and biological molecules. Solid-state 17O (spin-5/2) NMR has, however, remained largely unexplored due to experimental difficulties in detecting NMR signals for quadrupolar nuclei. Given the fact that numerous NMR studies have been carried out for 1H, 13C and 15N,17O can be considered as the last frontier of biomolecular NMR spectroscopy. In the past several years, we have developed a comprehensive research program on solid-state 17O NMR studies of organic and biological compounds. We have first synthesized a series of 17O-labeled compounds containing various functional groups, and then characterized their solid-state 17O NMR properties. Most of the functional groups that we examined had no solid-state 17O NMR data available before our studies. We have also applied for the first time 17O multiple-quantum magic-angle spinning (MQMAS) technique to organic compounds and obtained high-resolution 17O NMR spectra where different oxygen sites can be resolved. The most intriguing aspect of 17O NMR is the remarkable sensitivity of 17O NMR parameters (both chemical shift and quadrupole coupling tensors) to hydrogen bonding interaction. We are currently expanding our effort in the development and application of solid-state 17O NMR with an emphasis on biological molecules.
Our publications in this area
- I. C. M. Kwan, X. Mo, and G. Wu, Journal of the American Chemical Society 129, 2397-2409 (2007).
- R. Ida, M. De Clerk, and G. Wu, Journal of Physical Chemistry Part A 110, 1065-1071 (2006).
- G. Wu and K. Yamada, Solid State Nuclear Magnetic Resonance 24, 196-208 (2003).
- G. Wu, S. Dong, R. Ida, and N. Reen, Journal of the American Chemical Society 124, 1768-1777 (2002).
- G. Wu and S. Dong, Journal of the American Chemical Society 123, 9119-9125 (2001).
- G. Wu, S. Dong, and R. Ida, Chemical Communications 891-892 (2001).
- S. Dong, R. Ida, and G. Wu, Journal of Physical Chemistry Part A 104, 11194-11202 (2000).
- K. Yamada, S. Dong, and G. Wu. Journal of the American Chemical Society 122, 11602-11609 (2000).
- G. Wu, A. Hook, S. Dong, and K. Yamada. Journal of Physical Chemistry Part A 104, 4102-4107 (2000).
- G. Wu, K. Yamada, S. Dong, and H. Grondey. Journal of the American Chemical Society 122, 4215-4216 (2000).
- S. Dong, K. Yamada, and G. Wu. Z. Naturforsch. 55A, 21-28 (2000).
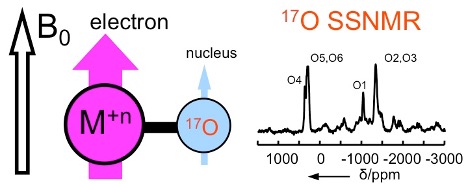
NMR studies of paramagnetic solids can be traced back to the pioneering works by Knight on metals in 1949 and by Bloembergen on CuSO4·5H2O crystals in 1950. In paramagnetic systems, the very strong interactions between unpaired electrons and nuclear spins known as the hyperfine coupling tensors (A-tensors) often present difficulties for NMR studies, because they cause not only significant shifts in NMR signals but also result in broad signals, which in many cases can be too broad to be detectable by NMR. As a result, electron paramagnetic resonance (EPR) spectroscopy is always the method of choice for measuring A-tensors in paramagnetic compounds. In this project, we explore the possibility of using modern solid-state NMR techniques to experimentally measure A-tensors for ligand atoms that are directly bonded to the paramagnetic center.
Our recent publications in this area
1. Y. Dai, V. Terskikh, and G. Wu, A Combined Solid-State 1H, 13C, 17O NMR and Periodic DFT Study of Paramagnetic Copper(II) Compounds. Solid State Nucl. Magn. Reson. 132, 101945 (2024).
2. L. Bauder and G. Wu, Solid-State 35/37Cl NMR Detection of Chlorine Atoms Directly Bound to Paramagnetic Cobalt(II) Ions in Powder Samples. Magn. Reson. Chem. 62, 145–155 (2024).
3. X. Kong, V. V. Terskikh, R. L. Khade, L. Yang, A. Rorick, Y. Zhang, P. He, Y. Huang, and G. Wu, Solid-State 17O NMR of Paramagnetic Coordination Compounds. Angew. Chem. Int. Ed. 54, 4753–4757 (2015).
Although advancements in solid-state NMR spectroscopy have significantly improved the study of quadrupolar nuclei in solid materials, investigating these nuclei in solution remains a formidable challenge—particularly within biological macromolecules like enzymes functioning in aqueous physiological environments.
Quadrupole Central Transition (QCT) NMR presents a promising solution to this problem. This technique utilizes the unique relaxation properties of half-integer quadrupolar nuclei in molecules undergoing slow isotropic tumbling motion. According to Redfield's relaxation theory, under such conditions, the central transitions (mI = +1/2 ↔ −1/2) should exhibit relatively long transverse relaxation times (T2), resulting in narrower spectral lines and thus enhanced NMR resolution.
Our research aims to advance QCT NMR techniques for the detection and analysis of various quadrupolar nuclei such as 23Na (I = 3/2), 39K (I = 3/2), 35Cl (I = 3/2), 17O (I = 5/2), 25Mg (I = 5/2), 67Zn (I = 5/2) and 59Co (I = 7/2) in solution, thereby establishing it as a powerful tool for probing biological macromolecules where molecular tumbling is naturally slow.
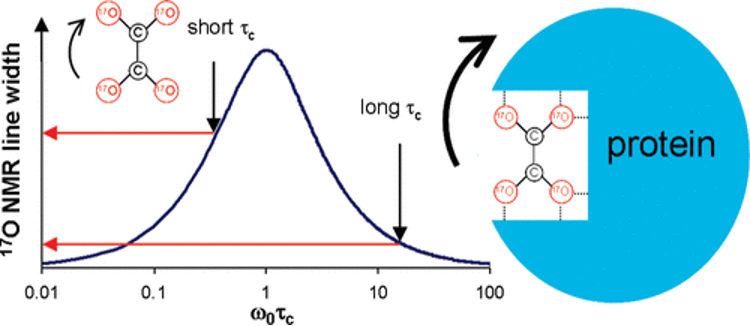
Our recent publications in this area
- Zhu, J.; Kwan, I. C. M.; Wu, G. Quadrupole-Central-Transition 17O NMR Spectroscopy of Protein-Ligand Complexes in Solution. J. Am. Chem. Soc. 2009, 131 (40), 14206–14207.
- Zhu, J.; Wu, G. Quadrupole Central Transition 17O NMR Spectroscopy of Biological Macromolecules in Aqueous Solution. J. Am. Chem. Soc. 2011, 133 (4), 920–932.
- Zhu, J.; Ye, E.; Terskikh, V.; Wu, G. Experimental Verification of the Theory of Nuclear Quadrupole Relaxation in Liquids over the Entire Range of Molecular Tumbling Motion. J. Phys. Chem. Lett. 2011, 2 (9), 1020–1023.
- Wu, G. An Approximate Analytical Expression for the Nuclear Quadrupole Transverse Relaxation Rate of Half-Integer Spins in Liquids. J. Magn. Reson. 2016, 269, 176–178.
- Shen, J.; Terskikh, V.; Wu, G. Observation of the Second-Order Quadrupolar Interaction as a Dominating NMR Relaxation Mechanism in Liquids: The Ultraslow Regime of Motion. J. Phys. Chem. Lett. 2016, 7 (17), 3412–3418.
- Shen, J.; Terskikh, V.; Wang, X.; Hung, I.; Gan, Z.; Wu, G. A Quadrupole-Central-Transition 17O NMR Study of Nicotinamide: Experimental Evidence of Cross-Correlation between Second-Order Quadrupolar Interaction and Magnetic Shielding Anisotropy. J. Phys. Chem. B 2018, 122 (18), 4813–4820.
- Shen, J.; Terskikh, V.; Wu, G. A Quadrupole-Central-Transition 59Co NMR Study of Cobalamins in Solution. ChemPhysChem 2019, 20 (2), 268–275.
