Gang Wu
Professor of Chemistry
Office: CHE408
Email: gang.wu@chem.queensu.ca
Phone: 613-533-2644
Affiliation: Physical, Faculty
Address: Chernoff Hall, CHE408 (office), CHE438 (lab)
Department of Chemistry
90 Bader Lane
Queen's University
Kingston, Ontario K7L 3N6
Lab CHE 438 Tel: 613-533-6000 ext. 75018
Research Interest:
Our primary research interests are concerned with the development of nuclear magnetic resonance (NMR) techniques in studying molecular structure, dynamics, and chemical bonding in chemically and biologically important systems. Currently, we are focused on new NMR methods for detecting 17O NMR signals in organic and biological molecules as well as in live cells. So why do we care about 17O NMR?
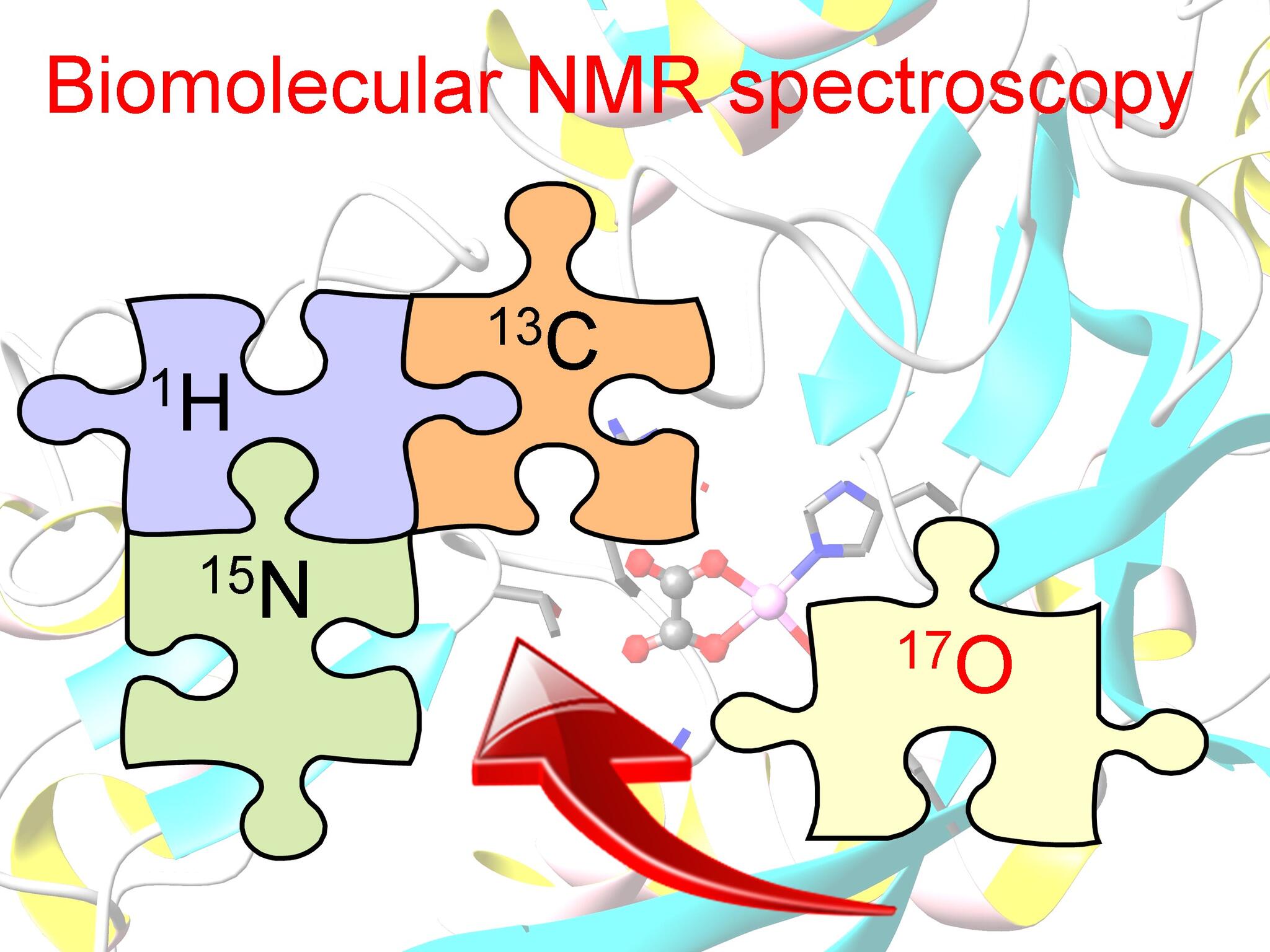
Most chemists agree that NMR has revolutionized, over the past six decades, not only chemical science but also other disciplines such as molecular biology and medical diagnostics. However, nearly all of the successful NMR applications to date have relied on the detection of atomic nuclei with the smallest non-zero nuclear spin number (I = 1/2) (i.e., 1H, 13C, 15N, and 31P). Any second-year chemistry undergraduate student, after having taken my course on “Organic Spectroscopy”, must have the following question lingering in mind but was afraid of asking: “Why didn’t we learn any NMR about oxygen, since it is among the most abundant elements found in organic and biological molecules?” Indeed, the oxygen element remains the only one that has not been readily accessible by NMR. So why not? This is primarily because oxygen has only one stable isotope that is NMR-active, 17O. This particular isotope is exceedingly hard to find in nature (natural abundance = 0.037%). In comparison, 1H is everywhere (natural abundance = 99.9%). Furthermore, this isotope of oxygen has an unusual nuclear spin number (I = 5/2). Any atomic nucleus with I > 1/2 is called being “quadrupolar” (or “naughty” in layman’s language). The reason quadrupolar nuclei are “naughty” is that they often give rise to very broad NMR signals. That is, it is difficult to extract any useful chemical information from their NMR spectra.
Now if we view organic and biological molecules as jigsaw puzzles made out of four kinds of pieces (H, C, N, and O elements), so far chemists are solving molecular puzzles without the capability of “seeing” one quart of the O pieces! The goal of our current research is to make these missing O pieces “visible”. Would that make the molecular game a little easier?